Nature of Electricity

Contents
- Nature of Electricity Definition: Electricity is defined as the flow of electrons through a conductor due to an electrical potential difference.
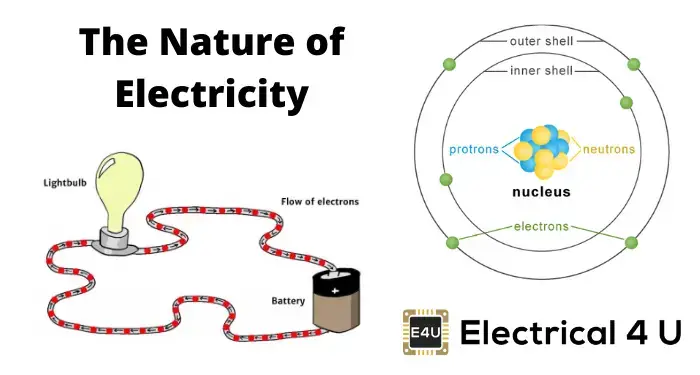
- Atomic Structure: An atom is made of a nucleus with protons and neutrons, surrounded by electrons.
- Free Electrons: Electrons that are loosely bonded and can move from one atom to another are called free electrons.
- Conductors: Materials with many free electrons, like copper and aluminum, are good conductors of electricity.
- Insulators: Materials with few free electrons, like glass and mica, are poor conductors of electricity.
Nature of Electricity and Concept of Electricity
Electricity is the most common form of energy. We use it for lighting, transportation, cooking, communication, and making products in factories. To understand the nature of electricity, we need to look at the structure of matter. All substances are made of tiny particles called molecules, which are the smallest units retaining the substance’s properties. Molecules are made of atoms, the smallest particles of an element.
There are two types of substances: elements and compounds. Elements have molecules made of similar atoms. Compounds have molecules with different kinds of atoms. Understanding electricity involves studying the atomic structures of these substances.
Structure of Atom
An atom consists of one central nucleus. The nucleus is made up of positive protons and charge less neutrons. This nucleus is surrounded by numbers of orbital electrons. Each electron has a negative charge of – 1.602 × 10– 19 Coulomb and each proton in the nucleus has a positive charge of +1.602 × 10 – 19 Coulomb. Because of the opposite charge there is some attraction force between the nucleus and orbiting electrons. Electrons have relatively negligible mass compared to the mass of the nucleus. The mass of each proton and neutrons is 1840 times the mass of an electron.

As the modulus value of each electron and each proton are same, the number of electrons is equal to the number protons in an electrically neutral atom. An atom becomes positively charged ion when it loses electrons and similarly an atom becomes negative ion when it gains electrons.
Atoms may have loosely bonded electrons in their outer orbits, called free electrons. These electrons need very little energy to detach from their parent atom and move randomly, transferring from one atom to another. A substance with an unequal number of electrons and protons is electrically charged. More electrons mean it is negatively charged, and more protons mean it is positively charged.

The basic nature of electricity is that when a negatively charged body connects to a positively charged body through a conductor, excess electrons flow from the negative to the positive body to balance the electron shortage.
Hope you got the very basic concept of electricity from the above explanation. There are some materials which have plenty of free electrons at normal room temperature. Very well known examples of this type of materials are, silver, copper, aluminium, zinc etc. The movement of these free electrons can easily be directed to a particular direction if the electrical potential difference is applied across the piece of these materials. Because of plenty of free electrons these materials have good electrical conductivity. These materials are referred as good conductor. The drift of electrons in a conductor in one direction is known as the current. Actually electrons flow from lower potential (-Ve) to higher potential (+Ve) but the general conventional direction of current has been considered as the highest potential point to lower potential point, so the conventional direction of current has been just opposite of the direction of flow of electrons. In non-metallic materials, such as glass, mica, slate, porcelain, the outermost orbit is completed and there is almost no chance of loosing electrons from its outermost shell. Hence there is hardly any free electron present in this type of material.
Hence, these materials cannot conduct electricity in other words electrical conductivity of these materials is very poor. Such material are known as non-conductor or electrical insulator. The nature of electricity is to flow through a conductor while an electrical potential difference applied across it, but not to flow through insulator even high electrical potential difference applied across them.